|
Fujicalin« is a unique patented form of Dibasic Calcium Phosphate Anhydrous (DCPA). It is designed to function as a direct compression excipient and has exceptional flow and compression characteristics, while maintaining the ability for rapid disintegration. Fujicalin« is spherically granulated, has lower mean particle size and extremely high specific surface area when compared to other available DCPA and Dibasic Calcium Phosphate Dihydrate (DCPD). |
|
|
|
|
|
PROPERTY |
DCPA |
DCPD |
|
|
Fujicalin« |
Conventional |
||
|
Mean particle size (Ám) |
115 |
43 |
127 |
|
Bulk density (g/ml) loose |
0.42 |
0.76 |
0.83 |
|
Bulk density (g/ml) tapped |
0.45 |
0.78 |
0.91 |
|
Angle of repose (░) |
30 |
42 |
35 |
|
BET specific surface area (m2/g) |
40 |
1.95 |
0.57 |
|
Oil adsorption capacity (ml/g) |
1.1 |
0.4 |
0.2 |
|
Water adsorption capacity (ml/g) |
1.2 |
0.5 |
0.2 |
|
Loss on drying (%) |
0.5 |
-- |
2.8 |
|
Anhydrous form of
calcium phosphates are often used to overcome the problems related to DCPD.
For example, water of crystallization could possibly react with hydrolysable
drugs during processing, affecting stability of the tablet. Fujicalin« is anhydrous and spherically
granulated to solve your problems with hydrolysable as well as oily actives. |
|
|
|
Formulation: i. One kg of tocopherol acetate (Vitamin E) made to a
50% ethanol solution was mixed with 2 kg of Fujicalin«
in a vertical granulator at the rate of 50 ml/min. A dried powder was
obtained after evaporating the alcohol to dryness. |
|
|
|
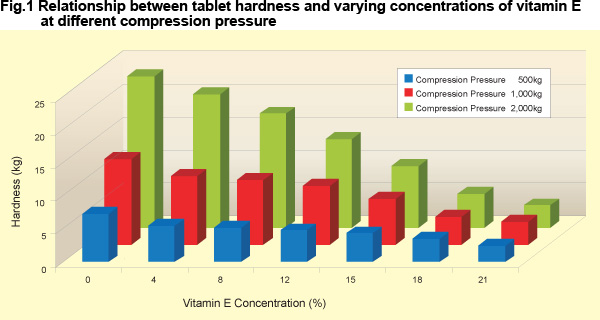
The hardness decreased with increase in Vitamin E concentration at all compression pressures tested. However, high quality Fujicalin« based compact tablets were possible with a Vitamin E load of up to 15% with this formulation. |
|
Dosage and Safety: |
|
If you would prefer
not to receive email newsletter, or you’ve changed your email address,
please click
here |