|
In the February issue technical newsletter, we introduced F-MELT®, a proprietary excipient system for oral disintegrating tablets (ODT). ODT is a rapidly developing market and more and more technologies are coming to the fore front with distinct advantages. However, direct compression is still the preferred choice because it uses conventional equipment and materials while maintaining lower manufacturing costs. F-MELT® is ideally suited for direct compression and in this
newsletter; we demonstrate F-MELT®
formulations with a focus on three important characters of ODT, viz. tablet
hardness, mouth feel and acceptable oral disintegration times. In some cases,
the desired tablet cannot be achieved with F-MELT®
alone. However, a higher tablet hardness and pleasant mouth feel can be
maintained by incorporating additional organic or inorganic excipients with F-MELT® as illustrated in the following
examples. |
|
|
|
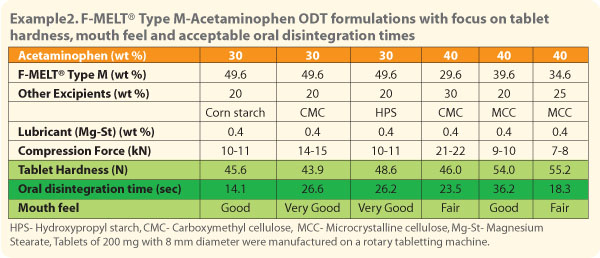
In case of acetaminophen and aspirin as API, a combination of CMC or Kollidon CL with F-MELT®Type C provided hard tablets with very good mouth feel and satisfactory oral disintegration times. |
|
|
|
In case of acetaminophen, a combination of corn starch with F-MELT®Type M provided hard tablets with good mouth feel and excellent oral disintegration time. |
|
|
|
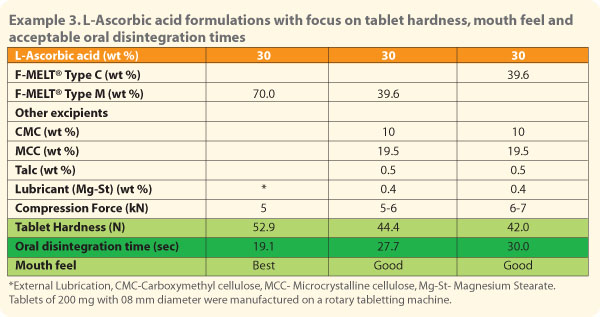
Ascorbic acid is a
difficult API for tabletting due to its sticky nature. F-MELT® could be successfully used in
formulations to achieve ODT of ascorbic acid. An external lubrication system
provided harder tablets with excellent ODT at 30% API load. |
|
|
|
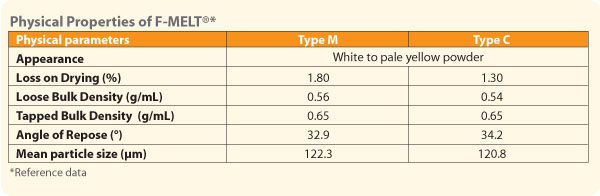
F-MELT® is a spray-dried powder of five pharmaceutical excipients consisting of carbohydrates, inorganic ingredients and disintegrants. It is available in two grades, F-MELT® Type C and F-MELT® Type M. F-MELT® Type C conforms to USP-NF, EP and JP and Type M conforms to USP-NF and JP An U.S. Drug Master file is available for Type C. |
|
|
|
If you would prefer
not to receive email newsletter, or you’ve changed your email address,
please click
here |