|
Sodium valproate has been widely used as an anti-epileptic agent to cure epilepsy and prevent epileptic stroke. Frequent administration of the tablet is often recommended to sustain blood plasma levels of sodium valproate. A slow and sustained release of the active ingredient will be beneficial to patients who need to maintain sustainable levels of sodium valproate in the blood plasma. Due to its
highly hygroscopic nature, sodium valproate formulations often poses problem
during production and storage. In this newsletter, we refer to a patent
published in |
|
Method of preparation of sustained release sodium valproate tablets |
|
Example: Sodium valproate (200 parts) dissolved
in water was absorbed on to Neusilin®
(97 parts) and dried in an oven to make a free flowing powder. This portion
was mixed with ethyl cellulose (45 parts) and 1,2-dichloroethane (100 parts).
The mixture was kneaded and homogenized before drying in an oven. Sodium
valproate tablets (345 mg, Ö 10 mm) were manufactured by direct
compression with a suitable lubricant (magnesium stearate, 3 parts). The
tablets were then film coated using suitable film coating materials. For
therapeutic use, the authors of the patent recommend 400-1,200 mg of sodium
valproate orally once or twice a day depending on the age and body weight of
the patient. |
|
Table
1. Dissolution rate of sodium valproate tablets
|
|||||||||||||||||||||||||||||||||||||||||
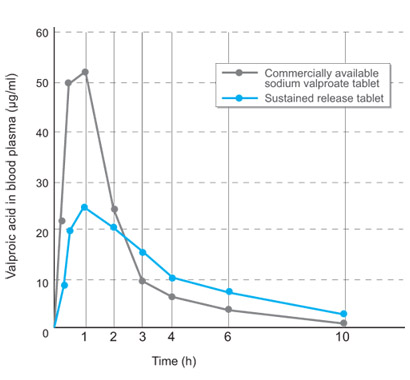
Male Beagle dogs
(body weight 11-14 kg) fasted overnight were orally administered 2 tablets
each. Both control and experimental batch received commercially available
sodium valproate tablets and sustained released tablets containing 200 mg
sodium valproate respectively. Blood sampling was performed at 0.25, 0.5, 1,
2, 3, 4, 6 and 10 h after administration and the valproic acid levels in
plasma were determined by gas chromatography. |
|||||||||||||||||||||||||||||||||||||||||
|
Stability tests |
|
The sustained release tablets packed in Press Through Package (PTP) with polyvinyl chloride and aluminum foil were stored at 40°C, 75% RH for 3months (accelerated stability tests). Appearance, hardness, content and dissolution tests were carried out to evaluate stability. Table 2. Accelerated stability test data of sodium valproate tablets
*t50; Hours needed for dissolving 50% of sodium valproate from the tablet |
|
Conclusions |
|
Neusilin® was successfully used in preparation of sustained release tablets of sodium valproate. The formulation overcame problems associated with stability of highly hygroscopic API like valproic acid as well as frequent administration to maintain sufficient levels of API in blood plasma. The success in this formulation can be attributed to the unique physical characteristics of Neusilin®. Large amounts of API can be loaded on to Neusilin® because of its highly porous nature and large surface area. Neusilin® is highly flowable, protects deliquescent drugs and enables the production of high quality tablets at lower compression forces. |
|
|
Chemical
formula: Al203.MgO.1.7SiO2.xH2O |
|
Dosage and Safety |
|
Neusilin® is extremely safe with no reports of
adverse reactions and is an accepted ingredient by the |
|
To obtain a sample or to find your local
distributor, please contact us at pharma@fujichemical.co.jp.
For more technical information, please visit www.fujichemical.co.jp/english/neusilin.html |
|
If you would prefer not to receive email newsletter, or
you’ve changed your email address, please click here |