|
According to
several independent reports, nearly 40% of NCEs launched in US
market during the last decade are
poorly-water soluble APIs and that percentage continues to rise.
Consequently, many pharmaceutical companies spend a lot of time and effort to
improve solubility and increase bioavailability while trying to keep the
successful dosage form in mind. If on the other hand, outsourcing is an
option, consider the solid dispersion technique by Closed-cycle Spray Drying
(CSD) offered by Fuji Chemical Industry. Since 1999, our business
offers attractive “problem solving” services such as the
development and manufacture of amorphous API solid dispersions by CSD.
Our Expertise and Experience
Since 1999, Fuji
began the large scale solid dispersion manufacturing
by CSD technology. It was not an overnight transformation, but rather a
natural progression after 40 years of custom spray drying services for
pharmaceuticals. We believe this was part of the foundation for our success
as well as know-how to achieve solid dispersion proprietary technology and
quality of support. To date, we have solved issues at various stages of solid
dispersions such as formulation, process development and scale-up. At the
time of writing, we’ve completed roughly 30 projects covering the
following:

Working with You
from Bench to Scale
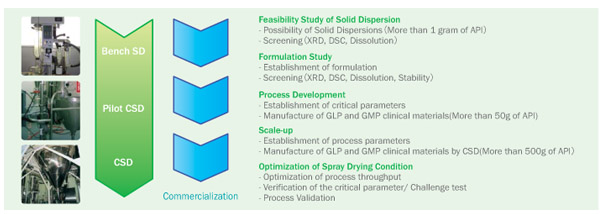
Successful
examples of Fuji Solid Dispersion Technology by CSD
Increase of
apparent solubility of API, the specific feature of solid dispersion, is the
driving force to enhance bioavailability. Fig 1 shows the apparent solubility
of the indomethacin/PVP solid dispersion increased approximately 6 times
compared to that of physical mixture. Furthermore, this super saturation
status is maintained for a long time. Fuji
designed solid dispersion also shows improved
bioavailability when compared to micronized API as indicated by the plasma
concentration of the API in rats (Fig2).
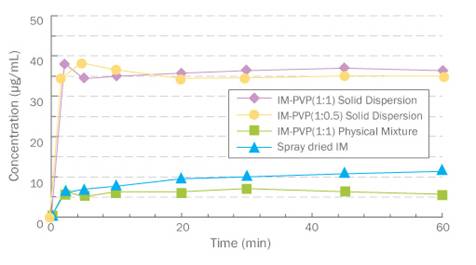
Fig. 1. Dissolution profile of Indomethacin
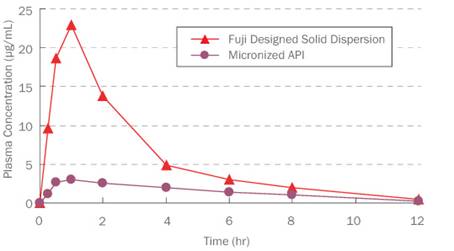
Fig. 2. Fuji
designed solid dispersion improves bioavailability
when compared to micronized API
Fuji Chemical
Industry is your Ideal Partner
In the field of
solid dispersion technology, Fuji
offers our customers unrivalled service and support
that includes the following:

Our seamless integration between process
development R&D and cGMP manufacturing enables us to provide customers
with flexible and rapid services
|