|
Neusilin®
US2 is a fine ultra
light granule of magnesium aluminometasilicate and is widely accepted as a
multifuntional excipient that improves the quality of pharmaceuticals. Due to
its large surface area and porous nature, US2 adsorbs high loads of oils or
water and can be mechanically compacted into high quality tablets.
Furthermore, |
|
|
|
|
|
PROPERTY |
Grade US2(Granule) |
|
Loss on Drying (%) |
1.4 |
|
Bulk Density - Loose (g/ml) |
0.15 |
|
Bulk Density - Tapped (g/ml) |
0.19 |
|
True Specific Gravity (g/ml) |
2.2 |
|
BET Specific Surface Area (m2/g) |
300 |
|
Mean Particle Size (ìm) (Agglomerate) |
60 - 120 |
|
Angle of Repose (Degrees) |
30 |
|
Oil Adsorbing Capacity (ml/g) |
3.2 |
|
pH of 5% Slurry |
7.4 |
|
Packaging (Kg) |
10 |
|
Among excipients, disintegrants play an improtant role in disintegration and dissolution of tablets. This factor is critical for drug absorption in vivo. In order to give the formulators the best choice of disintegrating agent in combination with Neusilin® US2, eight most common disintegrants were selected and their ability to quickly disintegrate compressed tablets was evaluated. |
|
|
|
Carboxy methyl starch sodium (Explotab) |
Croscarmellose sodium (Ac-Di-Sol) |
|
Corn starch |
Cross-link polyvinylpyrrolidone (Kollidon-CL) |
|
Carmellose calcium (ECG-505) |
Rice starch (Microperl) |
|
Hydroxy propyl cellulose (LH-21) |
Carboxy methyl cellulose (NS-300) |
|
|
|
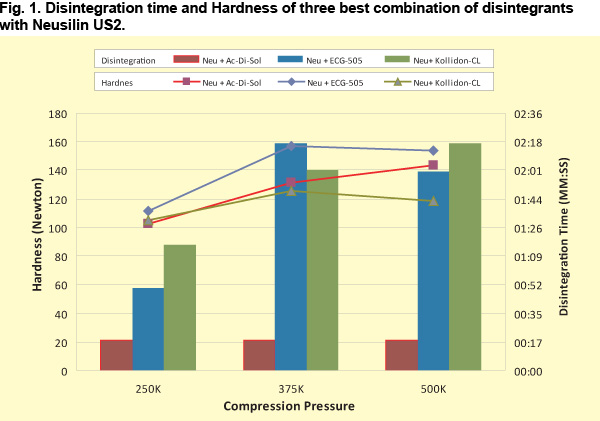
Neusilin® US2 was compounded with 5% disintegrant and 1% Magnesium stearate. The mixture was compressed into tablets of 11 mm dia and 200mg weight in a rotary tabletting machine. Disintegration test was carried out as per JP. To measure the disintegration time, one tablet is placed in each tube in the basket rack assembly containing six glass tubes, 7.75 cm long, open at the top and with a 1.8~2.2mm mesh, attached to the bottom. The assembly is positioned in a 1-liter beaker of water and maintained at 37°C. The basket rack is moved up and down at constant speed and the disintegration time was calculated as the time taken for the tablet to disintegrate and all the particles pass through the mesh screen. The results are summarized in the table given below. |
|
|
Hardness ( |
Tablet thickness (mm)at |
Disintegration time (min:sec) |
||||||
|
Compression Pressure |
250K |
375K |
500K |
250K |
375K |
500K |
250K |
375K |
500K |
|
Neusilin alone |
108.7 |
142.1 |
161.3 |
5.191 |
4.559 |
4.187 |
> 30:00 |
> 30:00 |
> 30:00 |
|
Neu* + Explotab |
95.9 |
130.1 |
144.8 |
5.064 |
4.478 |
4.12 |
> 30:00 |
> 30:00 |
> 30:00 |
|
Neu+ Corn Starch |
107.5 |
105.8 |
94.7 |
5.033 |
4.483 |
4.126 |
> 30:00 |
> 30:00 |
> 30:00 |
|
Neu + ECG-505 |
111.4 |
156.9 |
154 |
5.022 |
4.493 |
4.124 |
00:50 |
02:17 |
02:00 |
|
Neu+ LH-21 |
105.8 |
119.1 |
135.5 |
5.027 |
4.47 |
4.108 |
15:47 |
25:11 |
19:47 |
|
Neu + Ac-Di-Sol |
102.7 |
131.4 |
143.2 |
5.022 |
4.478 |
4.105 |
00:18 |
00:18 |
00:18 |
|
Neu+ Kollidon-CL |
105.1 |
125.3 |
118.7 |
5.078 |
4.514 |
4.151 |
01:16 |
02:01 |
02:17 |
|
Neu + Microperl |
94.8 |
115.4 |
134.3 |
5.084 |
4.501 |
4.131 |
>30:00 |
>30:00 |
>30:00 |
|
Neu + CMC |
97.8 |
128.9 |
144 |
5.103 |
4.496 |
4.133 |
26:52 |
18:30 |
18:45 |
|
*Neu=Neusilin |
|
|
|
Among the common disintegrants used, the most compatible disintegrant with Neusilin® US2 was found to be Croscarmellose sodium (Ac-Di-Sol) followed by Cross-link polyvinylpyrrolidone (Kollidon-CL) and Carmellose calcium (ECG-505). The Characteristics (large surface area and porus nature) of US2 and the cross linking of Croscarmellose sodium act synergestically allowing the tablet to swell and absorb many times it weight in water leading to quick disintegration. Neusilin® US2 improves flowability and make sufficiently hard tablets at low compression forces. Increase in hardness and compression pressure did not affect the disintegration time or tablet conformity when Croscarmellose sodium was used as a disintegrant. |
|
As most of the starch type disintegrants does not go well with Neusilin® US2, Croscarmellose sodium is your best choice when you choose Neusilin® US2 in your formulations. |
|
Dosage and Safety: |
|
If you would prefer
not to receive email newsletter, or you’ve changed your email address,
please click
here |